OUR RESEARCH
Viral structures and complexes
Many of the studies we have conducted require an understanding of the viral structures and their associated functions. For the work on the host ranges of CPV members of the lab (mainly Shwu Fen Chang) mapped the functions of the virus that controlled canine host range (the ability to infect canine cells in culture, or dogs) mapped to the capsid protein (ref)(ref). Studies with Michael Rossmann’s laboratory used X-ray crystallography to determine the structures of the capsids of CPV (ref)and then of the related FPV from cats (ref), which lacks the ability to infect dogs. Subsequently John Parker identified the transferrin receptor type-1 (TfR) as the receptor that was used for cell infection, and that also controlled the host range, as shown by Karsten Hueffer and Laura Palermo (ref)(ref)(ref).
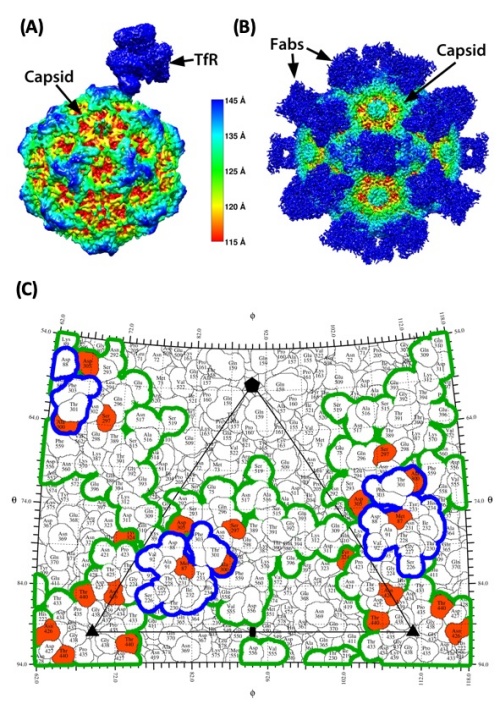
In parallel we were also examining the binding of antibodies to the CPV and FPV capsids, and showed that several different monoclonal antibodies bound to a variety of different overlapping epitopes on the surface of the capsid, as determined by escape mutant analysis conducted mainly by Lisa Strassheim and cryoEM microscopy conducted by Susan Hafenstein (ref)(ref).
More recently we have been able to use high resolution cryoEM in collaboration with Susan’s lab at Penn State University to determine the higher resolution structures of the TfR bound to the capsid of CPV, showing that the receptor bound through the top of the apical domain, and that two loops of the TfR bound across three loops of the capsid surface (ref).